Electrode Potential and Standard Electrode Potential
Electrode Potential and Standard Electrode Potential: Overview
This topic covers concepts, such as, Electrode Potential, Factors Affecting Electrode Potential of an Electrode/EMF of a cell, Standard Oxidation Electrode Potential & Standard Hydrogen Electrode etc.
Important Questions on Electrode Potential and Standard Electrode Potential
Two half-cell reactions of an electrochemical cell are given below :
What would be the final cell potential?
A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential measured is 0.422 V. Determine the concentration of silver ion in the cell. Given : .
An acidic solution of salt containing is electrolysed until all the copper is deposited. The electrolysis is continued for seven more minutes with the volume of solution kept at 100 mL and the current at 1.2 amp. The volume of gases evolved at NTP during the entire electrolysis:
A solution containing one mole per litre of each is being electrolysed by using inert electrodes. The values of standard electrode potentials in volts are :
With increasing voltage, the sequence of deposition of metals on the cathode will be :
The equilibrium constant for the reaction, would be, if the standard reduction potentials in acidic conditions are 0.77 V and 0.54 V respectively for couple.
The standard potential of the following cell is 0.23 V at and 0.21 V at .
(ii) Calculate for the cell reaction by assuming that these quantities remain unchanged in the range .
(iii) Calculate the solubility of AgCl in water at .
Given: The standard reduction potential of the couple is 0.80 V at .
A standard hydrogen electrode has zero electrode potential because
The emf of the cell:
at is Then the value of equilibrium constant for the cell reaction is:
Standard electrode potential data are useful for understanding the suitability of an oxidant in a redox titration. Some half cell reactions and their standard potentials are given below :
Identify the only incorrect statement regarding the quantitative estimation of aqueous
When the samples of copper with zinc impurity is to be purified by electrolysis, the appropriate electrodes are –
The electode potential of anode at standard condition is called standard _____ potential.
The standard oxidation potential of zinc is _____ .
The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called _____.(Cell emf/Cell potential)
The reaction is spontaneous if the cell potential is _____.(Positive/Negative)
The standard reduction potential for are respectively . The reaction will have more negative value when and are-
EMF of a cell in terms of reduction pontential of its left and right electrodes is:
The reaction is spontaneous if the cell potential is
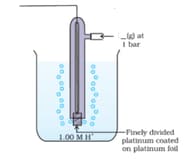
The IUPAC name of the gas released in the above diagram, where a blank is given is :
(Note : Fill the blanks by writing the chemical formulas in straightforward manner without using subscript. For example, the chemical formula of methane should be filled in the blank as and not as )
A reference electrode refers to an electrode that has a variable electrode potential.
cell of the following electrochemical cell is:
(Given )